Coupling Endergonic and Exergonic Reactions
Whereas some reactions could be catalysed by known enzymes. Mannich reactions the desired β-amino amide 4 was not detected.
9 6 Coupled Reactions Chemistry Libretexts
The reaction is ENDERGONIC or in the case of heat endothermic.

. This further underlines the unique character and orthogonality of the coupling reaction presented herein. Give an example of each Irreversible and Reversible Reactions. Also within the scope of bacterial metabolism is the study of the uptake and utilization of the inorganic or organic compounds required for growth and maintenance of a cellular steady state assimilation reactions.
The hypothesis proposes that early life may. Popper to publish his ideas. The assembly of NaI and PPh 3 with the RAE N -cyclohexanecarbonylphthalimide to form a charge transfer complex CTC via coulombic interaction is calculated to be exergonic by 38 kcal.
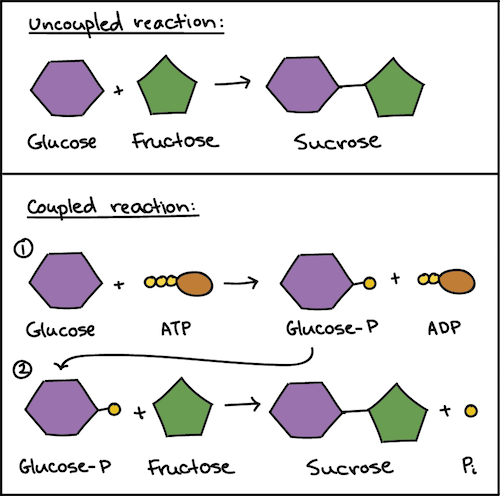
Based on thermodynamic considerations further exergonic reactions exist that could be exploited by microorganisms reactions 1526. Nevertheless these properties are sometimes desirable. The two reactions are coupled means -the energy provided by an exergonic reaction is either released Q.
We present here a review of the photochemical and electrochemical applications of multi-site proton-coupled electron transfer MS-PCET in organic synthesis. In inorganic reactions water is a common solvent dissolving many ionic compounds. The calculations also suggested that complexation of NaI and PPh 3 is exergonic in acetonitrile through the cation-π interaction exergonic by 46 kcalmol.
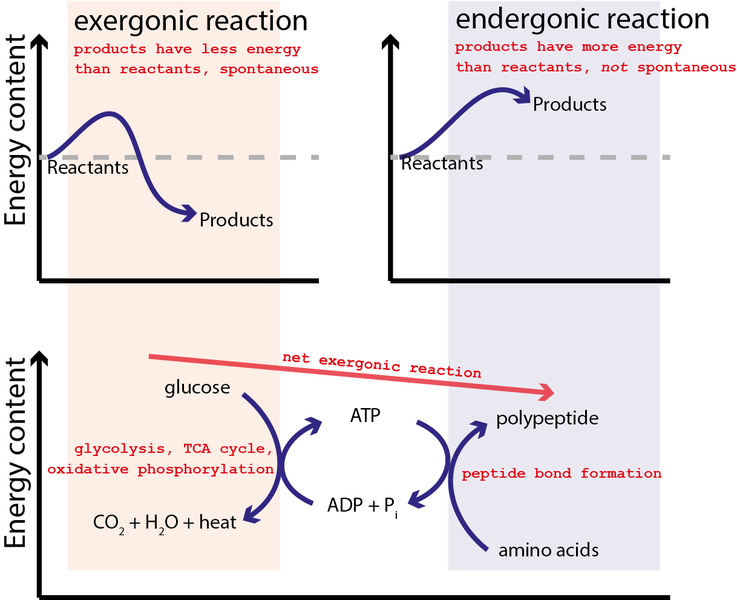
In the second reaction the formation of the bond between the carboxylic group at C-1 of 13-bisphosphoglycerate and orthophosphate occurs to form an anhydride called acyl phosphate. The reaction is quite exergonic with a G of -43 kJmol -103 kcalmol. The energy to phosphorylate ADP comes from catabolic reactions in the cell The ATP cycle is a revolving door through which energy passes during its transfer from catabolic to anabolic pathways Energy from catabolism exergonic energy-releasing processes Energy for cellular work endergonic energy-consuming processes ATP ADP P i H.
Cells the most fundamental and vital unit of life are found in all living things. This can be used to overcome endergonic reactions such as the. Although several examples of coupling reactions between α-bromo- and α-iododifluoroacetamides with alkenes have recently been reported.
CONCEPT 83âATP powers cellular work by coupling exergonic reactions to endergonic reactionsâ CONCEPT 84âEnzymes speed up metabolic reactions by lowering energy barriersâ CONCEPT 85âRegulation of enzyme activity helps control metabolismâ 9 Cellular Respiration and Fermentationâ Life Is Workâ. The reaction is quite endergonic with a G of 493 kJmol 118 kcalmol. These respective exergonic energy-yielding and endergonic energy-requiring reactions are catalyzed within the living bacterial.
This kind of reaction is termed EXERGONIC or if it releases heat exothermic. In organic reactions it is not usually used as a reaction solvent because it does not dissolve the reactants well and is amphoteric acidic and basic and nucleophilic. Thus this route is considered less feasible.
If on the other hand ΔG is positive and you are looking up at the hill then the reaction is unfavourable or not spontaneous ie. As such MS-PCET can function as a non-classical mechanism for. A quantitative measure of the favorability of a given reaction under these conditions is the change ΔG sometimes written delta G or dG in Gibbs.
The addition of the 4-aminopyridine-boryl radical to 1a is exergonic to give a. The ironsulfur world hypothesis is a set of proposals for the origin of life and the early evolution of life advanced in a series of articles between 1988 and 1992 by Günter Wächtershäuser a Munich patent lawyer with a degree in chemistry who had been encouraged and supported by philosopher Karl R. The products lend themselves to rapid derivatization Scheme 2BC.
MS-PCETs are redox mechanisms in which both an electron and a proton are exchanged together often in a concerted elementary step. According to the second law of thermodynamics for systems reacting at fixed temperature and pressure without input of non-Pressure Volume PV work there is a general natural tendency to achieve a minimum of the Gibbs free energy. ΔG 239 kcalmol and is endergonic by 121 kcalmol fig.
Cleavage of 3o bearing an indoline amide can easily be achieved through oxidative conversion to the.
Atp Cycle And Reaction Coupling Energy Article Khan Academy

What Atp Is And Why It S Important In Metabolism Molecules Chemistry Metabolism

Endergonic And Exergonic Reactions Feedback Inhibition Youtube

No comments for "Coupling Endergonic and Exergonic Reactions"
Post a Comment